Nathan Kilah, University of Tasmania
The words ‘chocolate’ and ‘disappointment’ don’t often go together.
But you may have experienced some disappointment if you’ve ever unwrapped the bright foil of an Easter egg to discover white, chalky chocolate inside. What is this white substance? Is it mould? Bacteria? Is it bad for you? Can you still eat it?!
The answer is yes, you can! It’s called “bloom” and it’s caused by fats or sugar from the chocolate. To understand why it forms, and how to avoid it forming, we need to consider the chemistry of chocolate.
The right stuff
Easter egg chocolate is made up of a relatively small number of ingredients: cacao beans, sugar, milk solids, flavourings, and emulsifiers to keep it all mixed together.
Fermenting and roasting cacao beans triggers many chemical reactions that develop delicious flavours. Much in the same way peanut butter can be made from peanuts, the roasted cacao beans are ground into a paste known as cocoa liquor.
The liquor is mixed with the other ingredients, and ground together with heating (known as conching) to form liquid chocolate.
Fat crystals
The fluidity of the cocoa liquor comes from the fats released when the beans are ground. These fat molecules are known as triglycerides, and they resemble the letter Y with three long zigzagging arms connected to a central junction. The triglyceride arms can vary, but they tend to be a mixture of saturated and unsaturated fatty acids.
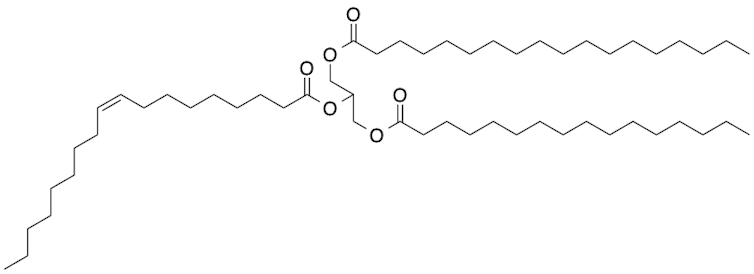
Author provided
When the melted chocolate cools, these triglyceride fats assemble into highly ordered structures that are crystals at the molecular scale. Depending on how well the temperature is controlled, the fats can take on one of six different crystal structures. These different crystal forms are called polymorphs.
Control your temper
The most desirable crystal form gives chocolate a smooth, glossy appearance, a clean snap and a melt-in-your-mouth texture. Achieving this requires careful temperature control from liquid to solid through a process known as ‘tempering’.
Poorly controlled cooling of the melted chocolate results in other crystal forms, which tend to have a less pleasing look and mouth feel – often chalky or gritty. These less desirable forms can convert during storage. And as the underlying crystal structure of the fats change, some of the triglycerides separate.
These separated fats collect at the surface as colourless crystals, giving the chocolate a white fat bloom. This is especially noticeable if the chocolate is poorly stored and goes through melting and resolidification.
The ingredients can also affect fat bloom. Cheap chocolate tends to use less cocoa butter and more milk solids, which introduce more saturated fats. Saturated fats are also common in nuts, and can migrate from the nut to the chocolate surface. So a chocolate-covered hazelnut is more likely to show fat bloom than a nut-free version.
Sugar or fat crystals?
Sugar bloom is less common than fat bloom, although they can look very similar. It occurs when sugar crystals separate from the chocolate, particularly under humid storage conditions.
You can tell the difference with a simple test. Sugar bloom will dissolve in a little water, while fat bloom will repel water and will melt if you touch it for a while. Unfortunately chocolate bloom can’t be reversed unless you completely melt the chocolate and recrystallise it at the correct temperature.
The easiest ways to avoid bloom on your Easter eggs is by choosing a brand with a high cocoa butter content, transporting and storing your eggs in a low temperature and humidity, and making sure you eat them before their best before date – assuming they last that long!
Nathan Kilah, Senior Lecturer in Chemistry, University of Tasmania
This article is republished from The Conversation under a Creative Commons licence. Read the original article.
If you enjoy our content, don’t keep it to yourself. Share our free eNews with your friends and encourage them to sign up.

