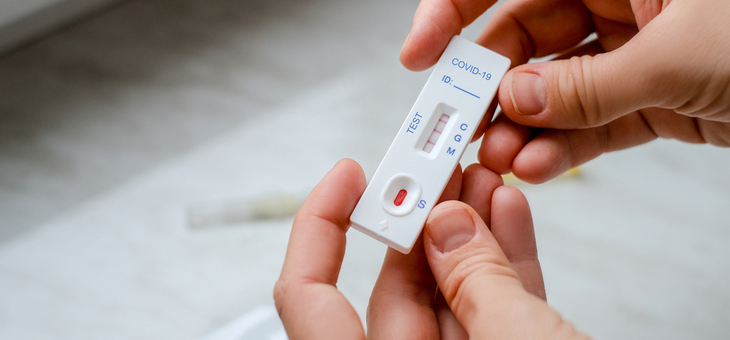
Two Australian medical tech companies have helped millions of people in Europe and North America out of lockdown through rapid COVID testing kits that can detect the virus in 10 minutes.
So, why isn’t the Australian government embracing this home-grown tech?
After a horror 2020 for the country, 2021 is turning out to be not much better. Sydney could be locked down until Christmas, Melbourne is in its sixth lockdown and other parts of NSW and Queensland are also in lockdown.
But then we look around the world and see Europe, North America and Asia starting to open up. Their vaccination programs are more advanced and many regions are using COVID-19 rapid antigen tests to quickly identify and isolate asymptomatic cases and stop outbreaks before they spread too far.
Vaccinating the population is obviously step one in bringing society back to some kind of normal. But even once vaccinated, we still need to detect and isolate cases as quickly as possible. The rapid testing kits are already being used by the Australian Defence Force and by mining companies for fly-in fly-out workers.
Read: Avalon Airport first to introduce COVID testing kiosks
Two Australian companies, Brisbane-based Ellume and Sydney’s Atomo, are leading manufacturers of the tests and have seen orders explode over the course of the pandemic – but so far Australian authorities have been reluctant to add the tests to its COVID arsenal.
Atomo chief executive John Kelly told The Australian he believes the problem may lie with vested interests in the medical technology industry.
“The groups that advise the government on diagnostic use in public health, they’re not called diagnostics NSW, they’re called Pathology NSW, right. I think that talks to the stakeholders,” Mr Kelly says.
State and federal governments have been relying on COVID tests being sent to laboratories. A result can take up to three days, meaning the highly infectious Delta variant can outpace contact tracers. Pathology lab testing is also much more expensive than the rapid tests.
“It’s a much greater cost. You could probably do five or six [rapid COVID tests] for the cost of one,” said Mr Kelly.
The Ellume tests were approved by the US Food and Drug Administration (FDA) in December and have played a supporting role to vaccines in allowing the country to open up over the past six months. The move was hailed as a turning point in the US fight against COVID-19.
Read: Australia’s four-phase COVID-normal. What we know so far
“Today’s authorisation is a major milestone in diagnostic testing for COVID-19,” FDA commissioner Dr Stephen Hahn said at the time.
“By authorising a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test and find out their results in as little as 20 minutes.”
Governments here claim a lack of data and level of accuracy as reasons the tests are not approved for wide use.
“Evaluation data on rapid antigen tests for COVID-19 are limited, and verification is required before use,” the Victorian Department of Health and Humans Services (VDHHS) guidelines say.
“The department recommends that health services, residential aged care facilities and other community services do not purchase these tests if approached by suppliers without consultation with the department.”
Read: Experts call for use of TGA-approved rapid COVID-19 tests
The federal health department agrees: “Rapid antigen tests can provide a result within 15-30 minutes. However, they have inferior analytical performance compared to the gold standard RT-PCR [lab-based test] for the diagnosis of COVID-19,” the department says.
“Further, there is considerable variability in the performance between the different rapid antigen tests.”
But Mr Kelly says the fears are overblown and the positives of the rapid tests greatly outweigh any potential negatives, and results internationally have shown that.
“The reality is, however, that it’s been well established overseas that regular antigen testing is a low cost and very effective way to screen asymptomatic people and manage risk because outbreaks are contained because people are protected very quickly,” Mr Kelly says.
“As the Delta variant has exploded in Australia and as the use of these tests has been validated overseas their [the governments’] rationale for not adopting these tests has become unsustainable.”
Do you think Australia should be sending our rapid COVID tests overseas? Do you think they could be our way out of lockdown? Let us know in the comments section below.
If you enjoy our content, don’t keep it to yourself. Share our free eNews with your friends and encourage them to sign up.